On December 31, 2020, China Sinopharm’s COVID-19 inactivated vaccine was approved by the State Food and Drug Administration to be listed with conditions. according to the existing data, its protection ratio was 79.34%, and it is an unity of security, effectiveness, accessibility and affordability.
China’s COVID-19 Vaccine Received Market Approval!
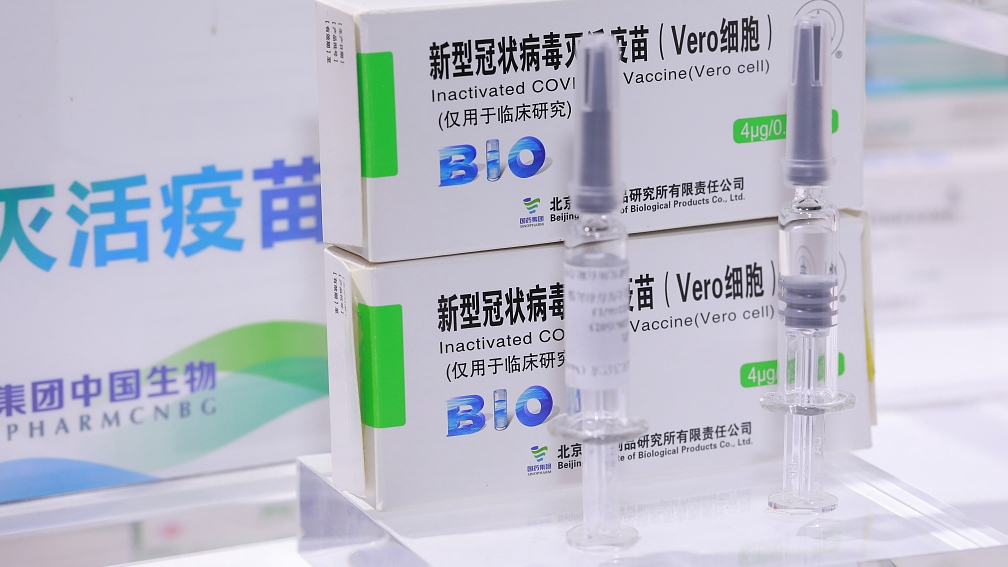
This is a great and good news for China and world. It will help and save million people’s life. The international economy will start to resume soon.
MAY BE YOU LIKE ALSO
©2015 szoumeide.com
甹lcp备09140074号









